MELANOTAN II
Peptide Data Sheet for Pharmacists and Compounding Professionals
BASIC INFORMATION
Name: Melanotan II (MT-II)
Class: Synthetic analog of alpha-melanocyte-stimulating hormone (α-MSH)
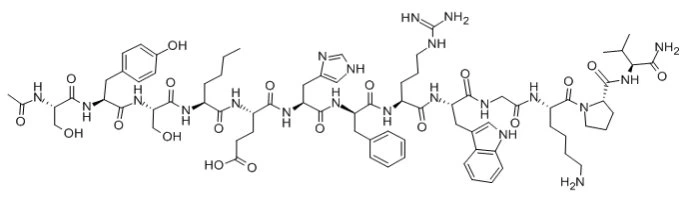
Structure: Cyclic heptapeptide analog of α-MSH
Molecular Weight: 1024.2 g/mol
Sequence: Ac-Nle-cyclo[Asp-His-D-Phe-Arg-Trp-Lys]-NH₂
Chemical Modifications:
- C-terminal amidation
- Contains non-natural amino acids (Nle, D-Phe)
Available Forms:
- Research peptide
- Not available as an FDA-approved medication
- Compounded formulations (subject to regulatory restrictions and safety concerns)
REGULATORY STATUS
FDA Status
- Not FDA-approved for any medical condition
- Classified as a research compound
- Warnings against its use have been issued from the US, UK, and several other countries due to safety concerns and lack of regulation.
- Not a legitimate dietary ingredient and cannot legally be used in dietary supplements.
Legal Considerations
- Unlicensed and largely untested for human use.
- Sale and distribution for human use are illegal in many jurisdictions.
- Often marketed “for research purposes only,” which does not make it safe or legal for human consumption.
- Compounding pharmacies should be aware of the significant safety concerns and lack of FDA approval.
MECHANISM OF ACTION
Melanotan II is a synthetic analog of α-MSH that:
- Non-selectively binds to and activates melanocortin receptors (MC1R, MC3R, MC4R, and MC5R).
- MC1R Activation: Stimulates melanocytes to produce melanin, leading to skin pigmentation (tanning).
- MC3R and MC4R Activation: Implicated in sexual function (increased libido, erectile function) and appetite regulation.
- MC5R Activation: Role less defined but involved in exocrine gland function.
Due to its non-selective binding, Melanotan II can affect multiple physiological systems beyond skin pigmentation.
PHARMACOKINETICS
| Parameter | Value | Notes | |———–|——-|——-| | Absorption | Rapid after subcutaneous injection | Bioavailability not well characterized | | Distribution | Limited human data available | Acts on melanocortin receptors in various tissues | | Metabolism | Likely enzymatic degradation | Specific pathways not well characterized | | Elimination | Half-life: Not well established in humans | Limited human pharmacokinetic data |
Note: Pharmacokinetic data is primarily derived from limited studies and animal research; human data is scarce.
POTENTIAL APPLICATIONS (INVESTIGATIONAL/UNAPPROVED)
Note: All applications are investigational and not FDA-approved. This information is provided for educational purposes only, highlighting areas of research and illicit use.
Primary Illicit Uses
- Skin Tanning: To achieve a tan without UV exposure.
- Sexual Dysfunction: To enhance libido and erectile function (related to PT-141, which is a metabolite).
Other Investigational Areas (Limited Evidence)
- Appetite suppression and weight loss
- Rosacea (theoretical, due to anti-inflammatory effects of α-MSH)
- Fibromyalgia (anecdotal)
ADMINISTRATION (ILLICIT/INVESTIGATIONAL)
Routes
- Subcutaneous injection (most common)
- Nasal spray (less common, variable absorption)
Investigational/Illicit Dosing
Note: No established safe or effective dosing regimen exists. The following information is based on unregulated use and research protocols and is not a recommendation for clinical use.
| Purpose | Investigational Dose Range | Frequency | |———|—————————-|———–| | Tanning | 0.025 mg/kg or 250-1000 mcg | Daily or every other day initially, then less frequently for maintenance | | Erectile Dysfunction | 0.025 mg/kg or 500-1000 mcg | As needed, prior to sexual activity |
SAFETY PROFILE
Reported Adverse Effects
Note: Safety data is limited and primarily from anecdotal reports, case studies of adverse events, and animal studies. Products are often unregulated and may be contaminated.
| System | Adverse Effects | |——–|—————-| | Gastrointestinal | Nausea (very common), vomiting, stomach cramps, decreased appetite | | Neurological | Headache, dizziness, fatigue, yawning, stretching complex | | Cardiovascular | Facial flushing, increased heart rate, palpitations, transient hypertension | | Dermatological | Darkening of existing moles, development of new moles, atypical melanocytic nevi, melanonychia (nail pigmentation), injection site reactions (pain, redness, bruising) | | Genitourinary | Spontaneous erections (priapism in some cases) | | Psychiatric | Mood changes, anxiety |
Potential Risks and Concerns
- Increased Risk of Melanoma: Significant concern due to stimulation of melanocytes and changes in moles.
- Rhabdomyolysis: Potentially fatal muscle breakdown (rare cases reported).
- Renal Infarction/Kidney Damage: Rare but serious cases reported.
- Encephalopathy Syndrome: Rare cases reported.
- Systemic Infections: Due to non-sterile injection practices or contaminated products.
- Unknown Long-Term Effects: Lack of rigorous clinical trials.
- Product Quality and Purity: Illicitly sourced products may be impure, incorrectly dosed, or contaminated.
Contraindications (Theoretical/Based on Risk Profile)
- History of melanoma or other skin cancers
- Atypical moles or numerous moles
- Cardiovascular disease
- Kidney disease
- Pregnancy and lactation
- Children and adolescents
- Psychiatric disorders
SPECIAL POPULATIONS
Pregnancy and Lactation
- No human data available.
- Strongly advised against use due to unknown risks and potential harm.
Pediatric
- No human data available.
- Strongly advised against use.
Geriatric
- No specific data available.
- Potentially higher risk of cardiovascular and other adverse effects.
PHARMACIST GUIDANCE
Compounding Considerations
- Not Recommended: Due to significant safety concerns, lack of FDA approval, and the availability of illicit, unregulated products, compounding Melanotan II is generally not advisable and may carry legal and ethical risks.
- Pharmacists should be aware of regulatory warnings and the potential dangers associated with this peptide.
- If legally permissible and ethically justifiable in a specific, highly controlled research context (not for general dispensing):
- Requires aseptic technique and sterile compounding environment.
- Adhere to USP <797> standards for sterile compounding.
Storage and Handling (Research Settings)
- Store lyophilized peptide at -20°C.
- Reconstituted solutions typically stored at 2-8°C.
- Use within a short period after reconstitution (e.g., 14-30 days, stability varies).
- Avoid repeated freeze-thaw cycles.
- Protect from light.
Patient Counseling Points (If a patient inquires or admits use)
- Emphasize Lack of Approval and Safety Data: Not FDA-approved for any indication; limited safety and efficacy data in humans.
- Highlight Serious Risks: Potential for skin cancer, cardiovascular events, kidney damage, and other severe side effects.
- Warn About Unregulated Products: Illicitly sourced products may be contaminated or incorrectly dosed.
- Discourage Use: Strongly advise against using Melanotan II for tanning or other unapproved uses.
- Recommend Consultation: Advise consultation with a healthcare provider for approved and safer alternatives for their concerns (e.g., sunless tanners, treatments for sexual dysfunction).
- Report Adverse Events: Encourage reporting of any adverse effects if they have used the product.
ETHICAL AND PROFESSIONAL CONSIDERATIONS
For Pharmacists
- Prioritize Patient Safety: Counsel patients about the dangers of Melanotan II.
- Adhere to Regulations: Understand and comply with all federal and state laws regarding unapproved drugs.
- Professional Integrity: Avoid any involvement in the illicit promotion or distribution of Melanotan II.
- Liability: Be aware of potential liability associated with unapproved and potentially harmful substances.
REFERENCES
- DermNet NZ. Melanotan II. https://dermnetnz.org/topics/melanotan-ii
- RxList. Melanotan-ii: Health Benefits, Side Effects, Uses, Dose & Precautions. https://www.rxlist.com/supplements/melanotan-ii.htm
- Evans-Brown M, et al. Use of melanotan I and II in the general population. BMJ. 2009;338:b566.
- Therapeutic Goods Administration (TGA), Australia. Safety alert: Melanotan-II injections. (Various alerts issued).
- Hjuler KF, Lorentzen HF. Melanoma associated with the use of melanotan-II. Dermatology. 2014;228:34–6.
- Nelson ME, et al. Melanotan II injection resulting in systemic toxicity and rhabdomyolysis. Clin Toxicol (Phila). 2012;50(10):1141-2.
