CJC-1295
Peptide Data Sheet for Pharmacists and Compounding Professionals
BASIC INFORMATION
Name: CJC-1295
Class: Growth hormone releasing hormone (GHRH) analog
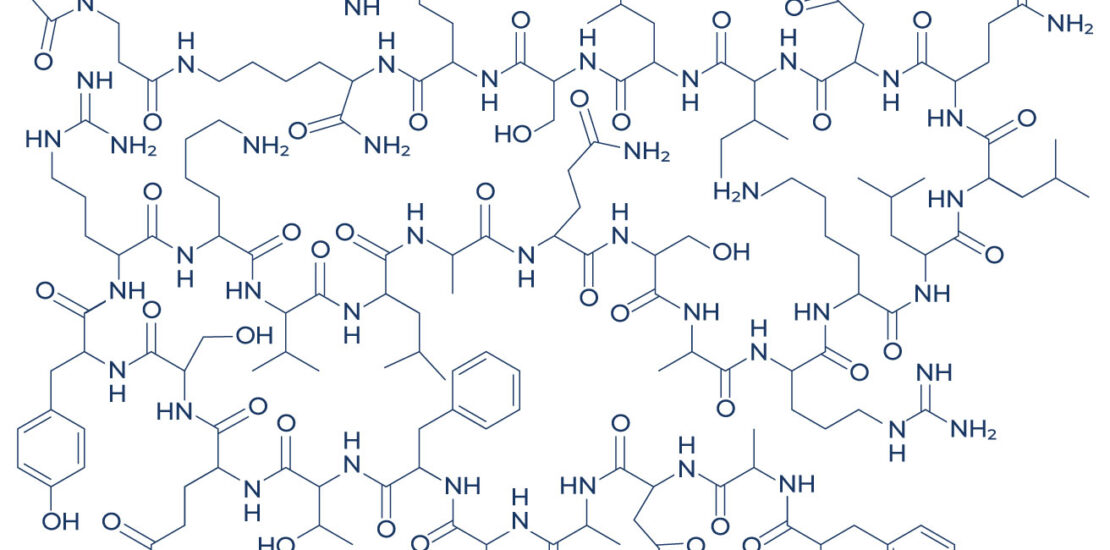
Structure: Modified 30-amino acid peptide analog of GHRH
Molecular Weight: 3368 g/mol
Chemical Modifications:
- Addition of Drug Affinity Complex (DAC)
- Substitution of four amino acids to prevent enzymatic degradation
- Lysine at position 30 allows for conjugation with biotin, albumin, or other proteins
Available Forms:
- Research peptide
- Compounded formulations (subject to regulatory requirements)
- Not available as an FDA-approved medication
- Often found in two variants:
- CJC-1295 with DAC (longer half-life)
- CJC-1295 without DAC (sometimes called “Modified GRF 1-29”)
REGULATORY STATUS
FDA Status
- Not FDA-approved for any medical condition
- Classified as a research compound
- FDA has clarified it is among peptides unlawful to use in making compounded medications
- Included on FDA’s list of substances nominated for the 503B bulks list that raised significant safety concerns
Legal Considerations
- Not approved for human use or consumption
- Often marketed “for research purposes only”
- Compounding pharmacies should be aware that FDA has specifically identified CJC-1295 as a substance that should not be used in compounding
MECHANISM OF ACTION
CJC-1295 is a synthetic analog of growth hormone-releasing hormone (GHRH) that:
- Binds to GHRH receptors in the anterior pituitary gland
- Stimulates the pulsatile release of growth hormone (GH)
- Increases insulin-like growth factor 1 (IGF-1) production
- With DAC modification, forms covalent bonds with serum albumin, extending its half-life
- Mimics the action of endogenous GHRH but with greater stability and duration
The DAC technology (Drug Affinity Complex) enables:
- Protection from enzymatic degradation
- Reduced renal clearance
- Extended half-life from minutes to days
PHARMACOKINETICS
| Parameter | Value | Notes | |———–|——-|——-| | Absorption | Rapid after subcutaneous injection | Complete bioavailability | | Distribution | Widely distributed | Binds to albumin (with DAC) | | Metabolism | Resistant to enzymatic degradation | Modified amino acids prevent breakdown | | Elimination | Half-life: <30 minutes (without DAC)<br>6-8 days (with DAC) | DAC significantly extends half-life |
Note: Pharmacokinetic data is primarily derived from limited human studies and animal research.
POTENTIAL APPLICATIONS (INVESTIGATIONAL)
Note: All applications are investigational and not FDA-approved. This information is provided for educational purposes only.
Endocrine
- Growth hormone deficiency (investigational)
- Age-related decline in growth hormone
Metabolic
- Body composition improvement
- Lipid metabolism
- Insulin sensitivity
Other Investigational Areas
- Recovery from injury
- Sleep quality improvement
- Immune system modulation
ADMINISTRATION (INVESTIGATIONAL)
Routes
- Subcutaneous injection (most common)
- Intramuscular injection (less common)
Investigational Dosing
Note: No established safe or effective dosing regimen exists. The following information is based on research protocols and is not a recommendation for clinical use.
| Variant | Investigational Dose Range | Frequency | |———|—————————-|———–| | CJC-1295 with DAC | 1-2 mg | Once weekly | | CJC-1295 without DAC | 100-200 mcg | Daily or multiple times weekly |
SAFETY PROFILE
Reported Adverse Effects
Note: Safety data is limited and primarily from small studies and anecdotal reports.
- Injection site reactions (redness, pain, swelling)
- Headache
- Flushing
- Water retention
- Numbness or tingling in extremities
- Joint pain
- Increased insulin resistance
- Fatigue
Potential Risks and Concerns
- Potential for growth promotion in pre-existing cancers
- Glucose metabolism alterations
- Fluid retention
- Carpal tunnel syndrome
- Gynecomastia
- Acromegalic effects with long-term use
- Unknown long-term effects
- Lack of quality control in commercially available products
Contraindications (Theoretical)
- Active malignancy
- History of pituitary disorders
- Uncontrolled diabetes
- Pregnancy and lactation
- Children and adolescents
- Intracranial hypertension
SPECIAL POPULATIONS
Pregnancy and Lactation
- No human data available
- Avoid use due to unknown risks
Pediatric
- No human data available
- Not recommended for use in pediatric populations
- Theoretical risk of affecting growth plates and development
Geriatric
- No specific data available
- Potentially higher risk of adverse effects
- May have different response due to age-related changes in GH/IGF-1 axis
PHARMACIST GUIDANCE
Compounding Considerations
- FDA has clarified that CJC-1295 is among peptides unlawful to use in making compounded medications
- Pharmacists should be aware of regulatory restrictions
- If compounding is legally permitted in specific circumstances:
- Requires aseptic technique and sterile compounding environment
- Stability affected by temperature and mechanical agitation
- Adhere to USP <797> standards for sterile compounding
Storage and Handling (Research Settings)
- Store lyophilized peptide at -20°C
- Reconstituted solutions typically stored at 2-8°C
- Use within 30 days of reconstitution
- Avoid repeated freeze-thaw cycles
- Protect from light
Patient Counseling Points
- Not FDA-approved for any indication
- Limited safety and efficacy data in humans
- Unknown long-term effects
- Potential for serious adverse effects
- Importance of discussing all supplements and medications with healthcare providers
- FDA has warned against its use in compounded medications
ETHICAL AND PROFESSIONAL CONSIDERATIONS
For Pharmacists
- Be aware of FDA position on CJC-1295 in compounding
- Understand legal and ethical implications of dispensing non-FDA approved peptides
- Maintain professional standards when discussing investigational compounds
- Provide evidence-based information when consulted about CJC-1295
- Consider liability issues related to non-FDA approved compounds
For Researchers
- Ensure proper informed consent in research settings
- Follow institutional and regulatory guidelines for research peptides
- Document and report adverse events
- Maintain scientific integrity in research protocols
REFERENCES
- FDA. Certain Bulk Drug Substances for Use in Compounding May Present Significant Safety Risks. https://www.fda.gov/drugs/human-drug-compounding/certain-bulk-drug-substances-use-compounding-may-present-significant-safety-risks
- Teichman SL, et al. Prolonged stimulation of growth hormone (GH) and insulin-like growth factor I secretion by CJC-1295, a long-acting analog of GH-releasing hormone, in healthy adults. J Clin Endocrinol Metab. 2006;91(3):799-805.
- Alba M, et al. Once-daily administration of CJC-1295, a long-acting growth hormone-releasing hormone (GHRH) analog, normalizes growth in the GHRH knockout mouse. Am J Physiol Endocrinol Metab. 2006;291(6):E1290-300.
- Jetté L, et al. Human growth hormone-releasing factor (hGRF)1-29-albumin bioconjugates activate the GRF receptor on the anterior pituitary in rats: identification of CJC-1295 as a long-lasting GRF analog. Endocrinology. 2005;146(7):3052-8.
- FDA. Pharmacy Compounding of Human Drug Products Under Section 503A of the Federal Food, Drug, and Cosmetic Act. https://www.fda.gov/media/94393/download
