BPC-157
Peptide Data Sheet for Pharmacists and Compounding Professionals
BASIC INFORMATION
Name: BPC-157 (Body Protection Compound-157)
Class: Synthetic pentadecapeptide
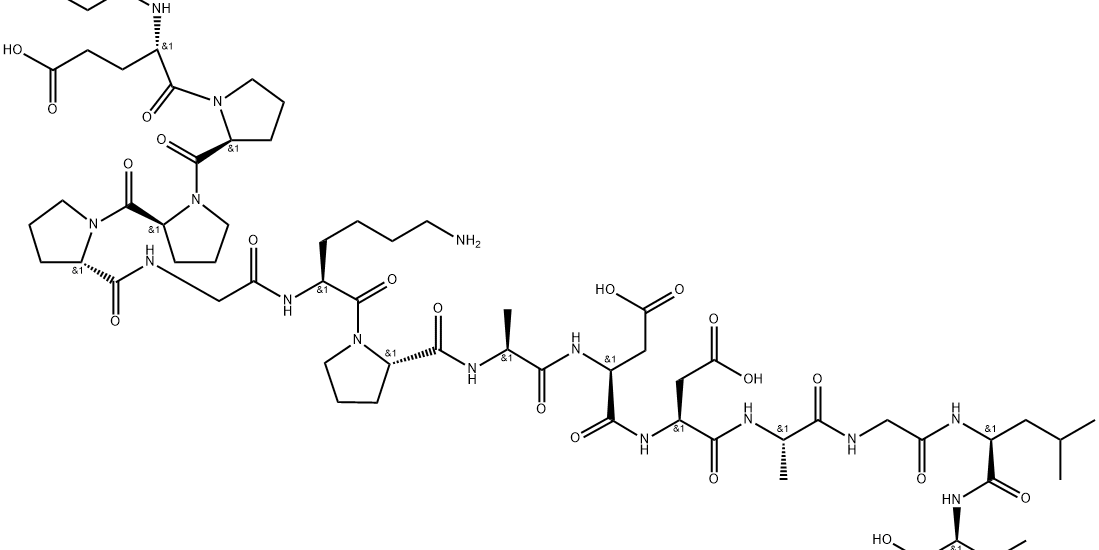
Structure: 15-amino acid partial sequence of Body Protection Compound (BPC)
Molecular Weight: 1419.53 g/mol
Sequence: Gly-Glu-Pro-Pro-Pro-Gly-Lys-Pro-Ala-Asp-Asp-Ala-Gly-Leu-Val
Chemical Modifications: None; direct partial sequence of body protection compound
Available Forms:
- Research peptide
- Compounded formulations (subject to regulatory requirements)
- Not available as an FDA-approved medication
REGULATORY STATUS
FDA Status
- Not FDA-approved for any medical condition
- Classified as a research compound
- FDA has clarified it is among peptides unlawful to use in making compounded medications
- Included on FDA’s list of bulk drug substances that present significant safety risks
Legal Considerations
- Not approved for human use or consumption
- Often marketed “for research purposes only”
- Compounding pharmacies should be aware that FDA has specifically identified BPC-157 as a substance that should not be used in compounding
MECHANISM OF ACTION
The precise mechanism of action remains under investigation, but research suggests BPC-157:
- Promotes angiogenesis (formation of new blood vessels)
- Accelerates the formation of granulation tissue
- Increases expression of growth hormone receptors
- Interacts with nitric oxide (NO) system
- May influence dopamine and serotonin systems
- Promotes tendon, ligament, and bone healing through:
- Enhanced fibroblast migration
- Increased type I collagen synthesis
- Activation of the FAK-paxillin pathway
PHARMACOKINETICS
| Parameter | Value | Notes | |———–|——-|——-| | Absorption | Variable based on administration route | Limited human data | | Distribution | Unknown | Primarily studied in animal models | | Metabolism | Likely proteolytic degradation | Specific pathways not well characterized | | Elimination | Unknown | Half-life not well established in humans |
Note: Pharmacokinetic data is primarily derived from animal studies; human data is extremely limited.
POTENTIAL APPLICATIONS (INVESTIGATIONAL)
Note: All applications are investigational and not FDA-approved. This information is provided for educational purposes only.
Gastrointestinal
- Inflammatory bowel disease
- Gastric ulcers
- Intestinal anastomosis healing
- Esophagogastric anastomosis
Musculoskeletal
- Tendon and ligament healing
- Muscle injury recovery
- Joint inflammation
- Bone healing
Neurological
- Peripheral nerve injury
- Traumatic brain injury
- Spinal cord injury
Other Investigational Areas
- Wound healing
- Corneal injury
- Periodontal tissue regeneration
- Cardioprotection
ADMINISTRATION (INVESTIGATIONAL)
Routes
- Subcutaneous injection
- Intramuscular injection
- Oral (stability issues, likely poor bioavailability)
- Topical (limited data on effectiveness)
Investigational Dosing
Note: No established safe or effective dosing regimen exists. The following information is based on research protocols and is not a recommendation for clinical use.
| Route | Investigational Dose Range | Frequency | |——-|—————————-|———–| | Subcutaneous | 250-500 mcg | Once or twice daily | | Intramuscular | 250-500 mcg | Once or twice daily | | Oral | 500-1000 mcg | Once or twice daily |
SAFETY PROFILE
Reported Adverse Effects
Note: Safety data is extremely limited and primarily from anecdotal reports and animal studies.
- Nausea
- Gastrointestinal discomfort
- Dizziness
- Injection site reactions
- Fatigue
- Headache
Potential Risks and Concerns
- Unknown long-term effects
- Potential for growth promotion in pre-existing cancers
- Lack of quality control in commercially available products
- Potential for contamination in non-pharmaceutical grade products
- Unknown drug interactions
- Unpredictable biological effects
Contraindications (Theoretical)
- Active malignancy
- Pregnancy and lactation
- Children and adolescents
- Autoimmune conditions
- Bleeding disorders
- Prior to surgical procedures
SPECIAL POPULATIONS
Pregnancy and Lactation
- No human data available
- Avoid use due to unknown risks
Pediatric
- No human data available
- Not recommended for use in pediatric populations
Geriatric
- No specific data available
- Potentially higher risk of adverse effects
PHARMACIST GUIDANCE
Compounding Considerations
- FDA has clarified that BPC-157 is among peptides unlawful to use in making compounded medications
- Pharmacists should be aware of regulatory restrictions
- If compounding is legally permitted in specific circumstances:
- Requires aseptic technique and sterile compounding environment
- Stability affected by temperature and mechanical agitation
- Adhere to USP <797> standards for sterile compounding
Storage and Handling (Research Settings)
- Store lyophilized peptide at -20°C
- Reconstituted solutions typically stored at 2-8°C
- Use within 2-4 weeks of reconstitution
- Avoid repeated freeze-thaw cycles
- Protect from light
Patient Counseling Points
- Not FDA-approved for any indication
- Limited safety and efficacy data in humans
- Unknown long-term effects
- Potential for serious adverse effects
- Importance of discussing all supplements and medications with healthcare providers
- FDA has warned against its use in compounded medications
ETHICAL AND PROFESSIONAL CONSIDERATIONS
For Pharmacists
- Be aware of FDA position on BPC-157 in compounding
- Understand legal and ethical implications of dispensing non-FDA approved peptides
- Maintain professional standards when discussing investigational compounds
- Provide evidence-based information when consulted about BPC-157
- Consider liability issues related to non-FDA approved compounds
For Researchers
- Ensure proper informed consent in research settings
- Follow institutional and regulatory guidelines for research peptides
- Document and report adverse events
- Maintain scientific integrity in research protocols
REFERENCES
- FDA. Certain Bulk Drug Substances for Use in Compounding May Present Significant Safety Risks. https://www.fda.gov/drugs/human-drug-compounding/certain-bulk-drug-substances-use-compounding-may-present-significant-safety-risks
- Chang CH, et al. Gastroprotective potential of pentadecapeptide BPC 157 against experimental gastric ulcers in rats. J Pharmacol Sci. 2011;115(4):547-55.
- Seiwerth S, et al. BPC 157 and standard angiogenic growth factors. Gastrointestinal tract healing, lessons from tendon, ligament, muscle and bone healing. Curr Pharm Des. 2018;24(18):1972-1989.
- Sikiric P, et al. Stable gastric pentadecapeptide BPC 157: novel therapy in gastrointestinal tract. Curr Pharm Des. 2011;17(16):1612-32.
- Gwyer D, et al. Gastric pentadecapeptide body protection compound BPC 157 and its role in accelerating musculoskeletal soft tissue healing. Cell Tissue Res. 2019;377(2):153-159.
